Introduction to Bonding
Chemical bonding describes a variety of interactions that hold atoms together in chemical compounds.
Answer: 1 📌📌📌 question Magnesium (Mg): 1s22s22p63s2 core electrons valence electrons - the answers to estudyassistant.com. This element has 6 energy levels and 8 valence electrons. This element is in the alkaline earth metals group and has 3 energy levels. This element is in the same family as Br, but has fewer protons than Mg.
Learning Objectives
List the types of chemical bonds and their general properties
Key Takeaways
Key Points
- Chemical bonds are forces that hold atoms together to make compounds or molecules.
- Chemical bonds include covalent, polar covalent, and ionic bonds.
- Atoms with relatively similar electronegativities share electrons between them and are connected by covalent bonds.
- Atoms with large differences in electronegativity transfer electrons to form ions. The ions then are attracted to each other. This attraction is known as an ionic bond.
Key Terms
- bond: A link or force between neighboring atoms in a molecule or compound.
- ionic bond: An attraction between two ions used to create an ionic compound. This attraction usually forms between a metal and a non-metal.
- covalent bond: An interaction between two atoms, which involves the sharing of one or more electrons to help each atom satisfy the octet rule. This interaction typically forms between two non-metals.
- intramolecular: Refers to interactions within a molecule.
- intermolecular forces: Refers to interactions between two or more molecules.
Chemical bonds
Chemical bonds are the connections between atoms in a molecule. These bonds include both strong intramolecular interactions, such as covalent and ionic bonds. They are related to weaker intermolecular forces, such as dipole-dipole interactions, the London dispersion forces, and hydrogen bonding. The weaker forces will be discussed in a later concept.
Chemical bonds: This pictures shows examples of chemical bonding using Lewis dot notation. Hydrogen and carbon are not bonded, while in water there is a single bond between each hydrogen and oxygen. Bonds, especially covalent bonds, are often represented as lines between bonded atoms. Acetylene has a triple bond, a special type of covalent bond that will be discussed later.
Covalent Bonds
Chemical bonds are the forces of attraction that tie atoms together. Bonds are formed when valence electrons, the electrons in the outermost electronic “shell” of an atom, interact. The nature of the interaction between the atoms depends on their relative electronegativity. Atoms with equal or similar electronegativity form covalent bonds, in which the valence electron density is shared between the two atoms. The electron density resides between the atoms and is attracted to both nuclei. This type of bond forms most frequently between two non- metals.
When there is a greater electronegativity difference than between covalently bonded atoms, the pair of atoms usually forms a polar covalent bond. The electrons are still shared between the atoms, but the electrons are not equally attracted to both elements. As a result, the electrons tend to be found near one particular atom most of the time. Again, polar covalent bonds tend to occur between non-metals.
Ionic Bonds
Finally, for atoms with the largest electronegativity differences (such as metals bonding with nonmetals), the bonding interaction is called ionic, and the valence electrons are typically represented as being transferred from the metal atom to the nonmetal. Once the electrons have been transferred to the non-metal, both the metal and the non-metal are considered to be ions. The two oppositely charged ions attract each other to form an ionic compound.
Bonds, Stability, and Compounds
Covalent interactions are directional and depend on orbital overlap, while ionic interactions have no particular directionality. Each of these interactions allows the atoms involved to gain eight electrons in their valence shell, satisfying the octet rule and making the atoms more stable.
These atomic properties help describe the macroscopic properties of compounds. For example, smaller covalent compounds that are held together by weaker bonds are frequently soft and malleable. On the other hand, longer-range covalent interactions can be quite strong, making their compounds very durable. Ionic compounds, though composed of strong bonding interactions, tend to form brittle crystalline lattices.
Ionic Bonds
Ionic bonds are a subset of chemical bonds that result from the transfer of valence electrons, typically between a metal and a nonmetal.
Learning Objectives
Summarize the characteristic features of ionic bonds
Key Takeaways
Key Points
- Ionic bonds are formed through the exchange of valence electrons between atoms, typically a metal and a nonmetal.
- The loss or gain of valence electrons allows ions to obey the octet rule and become more stable.
- Ionic compounds are typically neutral. Therefore, ions combine in ways that neutralize their charges.
Key Terms
- valence electrons: The electrons of an atom that can participate in the formation of chemical bonds with other atoms. They are the furthest electrons from the nucleus.
- octet rule: An atom is most stable when there are eight electrons in its valence shell.
Forming an Ion
Ionic bonds are a class of chemical bonds that result from the exchange of one or more valence electrons from one atom, typically a metal, to another, typically a nonmetal. This electron exchange results in an electrostatic attraction between the two atoms called an ionic bond. An atom that loses one or more valence electrons to become a positively charged ion is known as a cation, while an atom that gains electrons and becomes negatively charged is known as an anion.
This exchange of valence electrons allows ions to achieve electron configurations that mimic those of the noble gases, satisfying the octet rule. The octet rule states that an atom is most stable when there are eight electrons in its valence shell. Atoms with less than eight electrons tend to satisfy the duet rule, having two electrons in their valence shell. By satisfying the duet rule or the octet rule, ions are more stable.
A cation is indicated by a positive superscript charge (+ something) to the right of the atom. An anion is indicated by a negative superscript charge (- something) to the right of the atom. For example, if a sodium atom loses one electron, it will have one more proton than electron, giving it an overall +1 charge. The chemical symbol for the sodium ion is Na+1 or just Na+. Similarly, if a chlorine atom gains an extra electron, it becomes the chloride ion, Cl–. Both ions form because the ion is more stable than the atom due to the octet rule.
Forming an Ionic Bond
Once the oppositely charged ions form, they are attracted by their positive and negative charges and form an ionic compound. Ionic bonds are also formed when there is a large electronegativity difference between two atoms. This difference causes an unequal sharing of electrons such that one atom completely loses one or more electrons and the other atom gains one or more electrons, such as in the creation of an ionic bond between a metal atom (sodium) and a nonmetal (fluorine).
Formation of sodium fluoride: The transfer of electrons and subsequent attraction of oppositely charged ions.
Determining the Formula of an Ionic Compound
To determine the chemical formulas of ionic compounds, the following two conditions must be satisfied:
- Each ion must obey the octet rule for maximum stability.
- Ions will combine in a way that the overall ionic compound will be neutral. In other words, the charges of the ions must balance out.
Magnesium and fluorine combine to form an ionic compound. What is the formula for the compound?
Mg most commonly forms a 2+ ion. This is because Mg has two valence electrons and it would like to get rid of those two ions to obey the octet rule. Fluorine has seven valence electrons and usually forms the F – ion because it gains one electron to satisfy the octet rule. When Mg2+ and F – combine to form an ionic compound, their charges must cancel out. Therefore, one Mg2+ needs two F – ions to neutralize the charge. The 2+ of the Mg is balanced by having two -1 charged ions. Therefore, the formula of the compound is MgF2. The subscript two indicates that there are two fluorines that are ionically bonded to magnesium.
On the macroscopic scale, ionic compounds form crystalline lattice structures that are characterized by high melting and boiling points and good electrical conductivity when melted or solubilized.
Mg Valence Electrons
Example
Magnesium and fluorine combine to form an ionic compound. What is the formula for the compound?
Mg most commonly forms a 2+ ion. This is because Mg has two valence electrons and it would like to get rid of those two ions to obey the octet rule. Fluorine has seven valence electrons and as such, usually forms the F– ion because it gains one electron to satisfy the octet rule. When Mg2+ and F– combine to form an ionic compound, their charges must cancel out. Therefore, one Mg2+ needs two F– ions to balance. The 2+ of the Mg is balanced by having two -1 charged ions. Therefore, the formula of the compound is MgF2. The subscript two indicates that there are two fluorines that are ionically bonded to magnesium.
Covalent Bonds
Covalent bonding involves two atoms, typically nonmetals, sharing valence electrons.
Learning Objectives
Differentiate between covalent and ionic bonds
Key Takeaways
Key Points
- Covalent bonds involve two atoms, typically nonmetals, that share electron density to form strong bonding interactions.
- Covalent bonds include single, double, and triple bonds and are composed of sigma and pi bonding interactions where 2, 4, or 6 electrons are shared respectively.
- Covalent compounds typically have lower melting and boiling points than ionic compounds.
Key Terms
- electronegativity: The tendency of an atom or molecule to attract electrons and thus form bonds.
- single bond: A type of covalent bond where only two electrons are shared between atoms.
Forming Covalent Bonds
Covalent bonds are a class of chemical bonds where valence electrons are shared between two atoms, typically two nonmetals. The formation of a covalent bond allows the nonmetals to obey the octet rule and thus become more stable. For example:
- A fluorine atom has seven valence electrons. If it shares one electron with a carbon atom (which has four valence electrons), the fluorine will have a full octet (its seven electrons plus the one it is sharing with carbon).
- Carbon will then have five valence electrons (its four and the one its sharing with fluorine). Covalently sharing two electrons is also known as a “single bond.” Carbon will have to form four single bonds with four different fluorine atoms to fill its octet. The result is CF4 or carbon tetrafluoride.
Covalent bonding requires a specific orientation between atoms in order to achieve the overlap between bonding orbitals. Covalent bonding interactions include sigma-bonding (σ) and pi-bonding (π). Sigma bonds are the strongest type of covalent interaction and are formed via the overlap of atomic orbitals along the orbital axis. The overlapped orbitals allow the shared electrons to move freely between atoms. Pi bonds are a weaker type of covalent interactions and result from the overlap of two lobes of the interacting atomic orbitals above and below the orbital axis.
Mg Ion Valence Electrons
Covalent bonds can be single, double, and triple bonds.
- Single bonds occur when two electrons are shared and are composed of one sigma bond between the two atoms.
- Double bonds occur when four electrons are shared between the two atoms and consist of one sigma bond and one pi bond.
- Triple bonds occur when six electrons are shared between the two atoms and consist of one sigma bond and two pi bonds (see later concept for more info about pi and sigma bonds).
Ionic Compounds v. Molecular Compounds
Unlike an ionic bond, a covalent bond is stronger between two atoms with similar electronegativity. For atoms with equal electronegativity, the bond between them will be a non- polar covalent interaction. In non-polar covalent bonds, the electrons are equally shared between the two atoms. For atoms with differing electronegativity, the bond will be a polar covalent interaction, where the electrons will not be shared equally.

Ionic solids are generally characterized by high melting and boiling points along with brittle, crystalline structures. Covalent compounds, on the other hand, have lower melting and boiling points. Unlike ionic compounds, they are often not soluble in water and do not conduct electricity when solubilized.
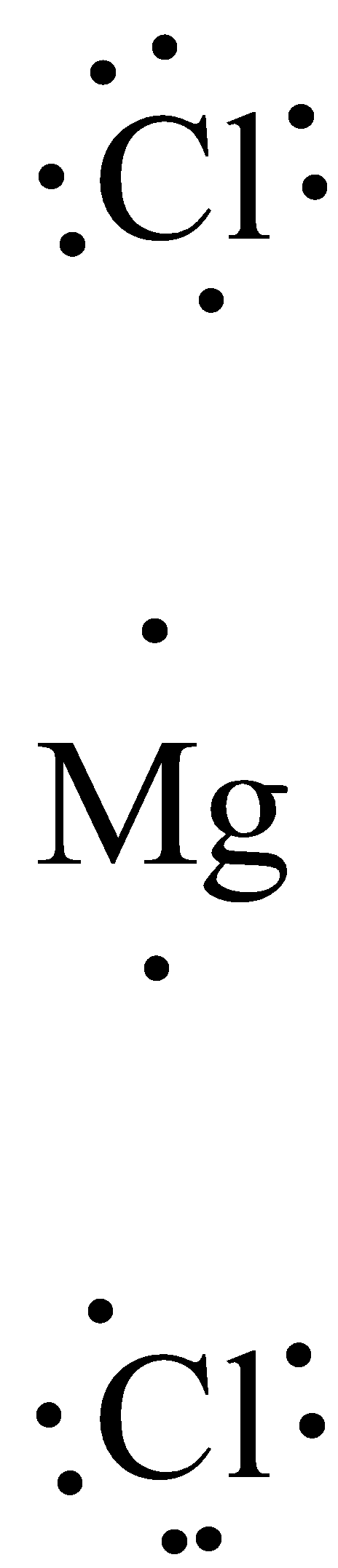
Learning Outcomes
- Determine the energy levels of electrons for the first 20 elements.
- Explain the relevance of valence electrons in chemical processes.
- Identify the number of valence electrons in an element.
- Describe the stability of an atom as a result of following the octet rule.
The structure of the atom was discussed in the previous unit and now we will focus on the role that electrons play in the formation of compounds. Regardless of the type of compound or the number of atoms or electrons involved, it is the electrons of those atoms that interact to form a compound.
Electron Arrangement
Electrons are not randomly arranged in an atom and their position within the atom can be described using electron arrangements, which are a simplified version of electron configurations. For each element of interest, we look at the number of electrons in a single atom and then determine how those electrons are arranged based on the atomic model. The main idea behind electron arrangements is that electrons can only exist at certain energy levels. By understanding the energy levels of electrons in an atom, we can predict properties and understand behavior of the atom.
As shown in the figure below, there are multiple energy levels where electrons can be found. As the energy level increases, the energy difference between them decreases. A maximum of two electrons can be found in the (n=1) level; eight electrons can be in the (n=2) level. I-o data network & wireless cards driver download for windows 10. Although the (n=3) and (n=4) levels show only eight electrons in this diagram, those energy levels can hold more but not until we start looking at the transition metals. We will only be concerned with the electron arrangements of elements through calcium (left( Z = 20 right)) so we will put a maximum of eight electrons in the (n=3) level and two in the (n=4) level.
Download icatch cameras. Example (PageIndex{1})
What is the electron arrangement of oxygen?
Jungo driver download for windows 10. Solution
Oxygen has eight electrons. The first two electrons will go in the (n=1) level. Two is the maximum number of electrons for the level so the other electrons will have to go in a higher energy level. The (n=2) level can hold up to eight electrons so the remaining six electrons will go in the (n=2) level. The electron arrangement of oxygen is (2, 6).
Example (PageIndex{2})
What is the electron arrangement of chlorine?
Solution
Chlorine has 17 electrons. The first two electrons will go in the (n=1) level. Two is the maximum number of electrons for the level so the other electrons will have to go in higher energy levels. The (n=2) level can hold up to eight electrons so the next 8 electrons will go in the (n=2) level. The remaining 7 electrons can go in the (n=3) level since it holds a maximum of 8 electrons. The electron arrangement of chlorine is (2, 8, 7).
The electron arrangement also provides information about the number of valence electrons. The valence electrons are the electrons in the highest energy level and the ones involved in ion and bond formation. Knowing the number of valence electrons will allow us to predict how a particular element will interact with other elements. Electrons in lower energy levels are called the core electrons.
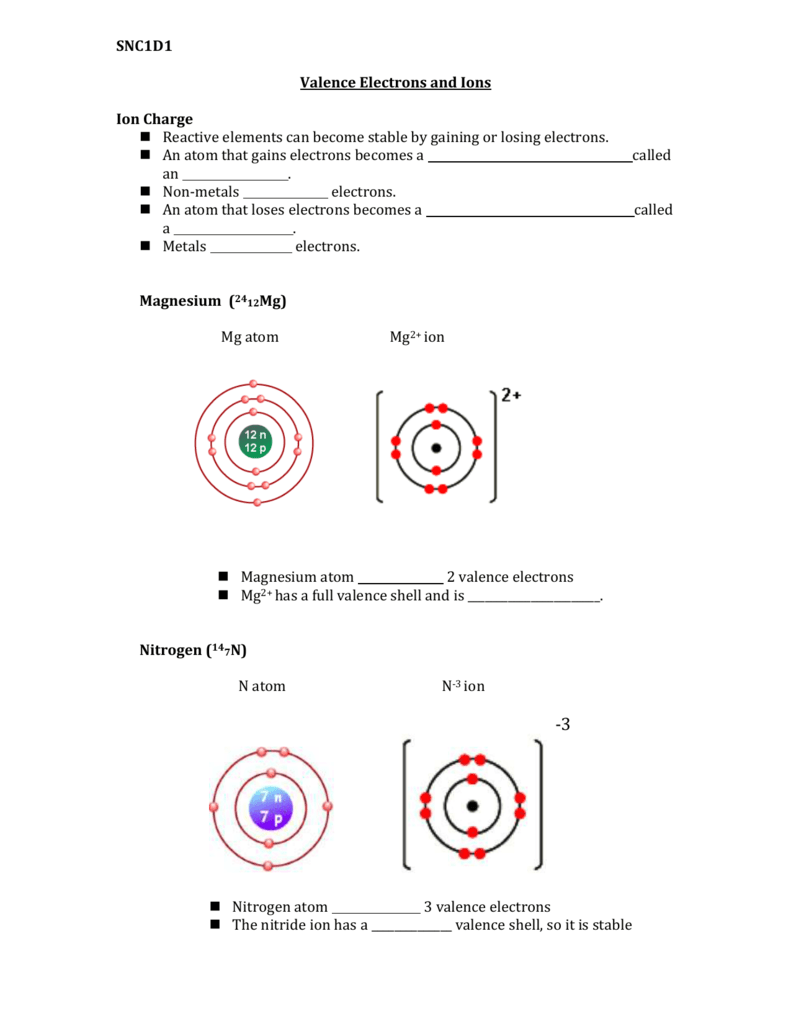
Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The first two electrons are found in the (n=1) energy level, the next eight electrons are found in the (n=2) level, and the remaining two electrons are found in the (n=3) level. The electrons always fill the lowest energy levels available until that level is filled, then electrons fill the next energy level until it is filled. This continues for all of the electrons in an atom. We can show the electron arrangement as (2, 8, 2) representing the electrons in the (n=1), (n=2), and (n=3) levels, respectively.
The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the (n=3) energy level which is the highest occupied energy level for magnesium. This corresponds to the (2+) charge formed when magnesium forms an ion. It is willing to lose 2 electrons so that it has the same electron arrangements as the nearest noble gas, which is neon (2, 8). Atoms will gain or lose electrons to look like the nearest noble gas because the noble gases are unreactive due to the stability of having eight electrons in the highest energy level. This desire of atoms to have eight electrons in their outermost shell is known as the octet rule.
Example (PageIndex{3})
Mg Has How Many Valence Electrons
What is the electron arrangement of aluminum? How many valence electrons does it have?
Solution
Aluminum has 13 electrons so it will have the electron arrangement (2, 8, 3) which represents two electrons in the (n=1) energy level, eight electrons in the (n=2) level, and three electrons in the (n=3) level. Aluminum has three valence electrons (indicated by the three electrons in the (n=3) level).
Example (PageIndex{4})
How many valence electrons does chlorine have? How many electrons will chlorine gain or lose to form an ion?
Solution
Chlorine has 7 electrons in its valence shell. To meet the octet rule, it must either gain one electron or lose seven electrons. Gaining one is easier than losing seven so it will gain one electron to have a total of eight electrons when it forms an ion (i.e. charged particle).
Contributors and Attributions
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)
